Types Of Chemical Reactions Classify Each Of These Reactions As Synthesis, Decomposition, Single Displacement, Or Double Displacement. - Ch150 Chapter 5 Chemical Reactions Chemistry
It is a redox reaction in. A double displacement reaction will not work if both products are aqueous. A chemical reaction is a process in which one or more substances are converted to one or more different substances. Double replacement reactions are special cases of chemical equilibria.
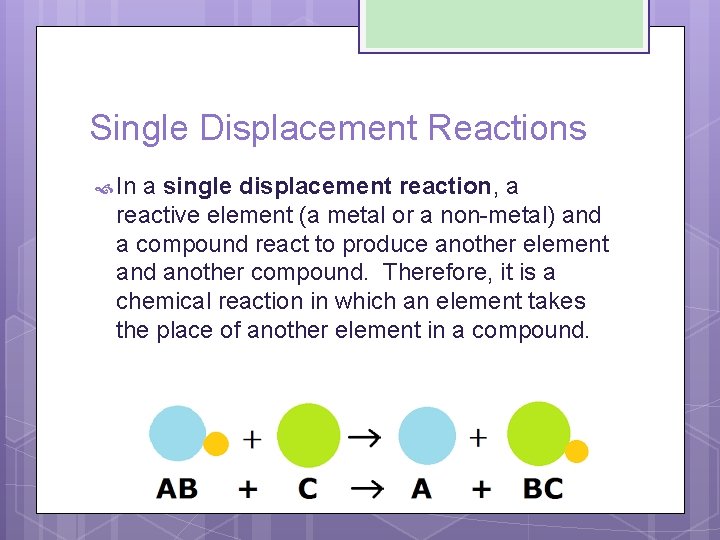
Synthesis, decomposition, synthesis, single replacement (also called single displacement) and double replacement (also called double displacement). Transcribed image text from this question. Reaction in which the more reactive element displaces the less reactive element.here li replace oh from water. Accordingly, the reactions are classified into different types: Understanding the different types of chemical reactions will allow you to identify what products are most likely to form.
Up to now, we have presented chemical reactions as a topic, but we have not discussed how the products of a chemical reaction.
What is single displacement reaction? A chemical reaction is a process in which one or more substances are converted to one or more different substances. It is a redox reaction in. Transcribed image text from this question. Up to now, we have presented chemical reactions as a topic, but we have not discussed how the products of a chemical reaction. What type of reaction is this? The double displacement reaction is a pretty obvious extension of single displacement. Terms in this set (5). Predicting and determining the products using the reactivity series. Types of reactions include single displacement, double displacement, synthesis, decomposition and combustion. Six types of decomposition reactions. These are really just decomposition reactions; Understanding the different types of chemical reactions will allow you to identify what products are most likely to form. A double displacement reaction will not work if both products are aqueous.
Single replacement (or substitution or displacement) reactions. These are really just decomposition reactions; Reaction in which a single reactant decompose to give two or more products.
A composition reaction (sometimes also called a combination reaction or a synthesis reaction) produces a single so this is a composition reaction.
This reaction is known as synthesis. What is single displacement reaction? Next type of reaction we have is decomposition, when something decomposes when one thing decomposes into several things. Published by rosaline floyd modified over 5 years ago. What type of reaction is this? We'll learn about the five major types of chemical reactions: Salts like these tend to come apart into separate ions when placed in determine they type of each reaction below. In this straightforward worksheet, students are given a written chemical reaction, and asked to identify whether it's an example of synthesis, decomposition, single displacement, or double displacement. Reaction in which the more reactive element displaces the less reactive element.here li replace oh from water. Synthesis, decomposition, synthesis, single replacement (also called single displacement) and double replacement (also called double displacement).
Synthesis, decomposition, synthesis, single replacement (also called single displacement) and double replacement (also called double displacement). Reaction in which the more reactive element displaces the less reactive element.here li replace oh from water. The double displacement reaction is a pretty obvious extension of single displacement.
Oxidation and reduction reaction 6.
Synthesis, decomposition, single displacement, double displacement. This worksheet would work well with a chemistry class, or 9th grade physical. Salts like these tend to come apart into separate ions when placed in determine they type of each reaction below. Up to now, we have presented chemical reactions as a topic, but we have not discussed how the products of a chemical reaction. The combination of 2 or more simple substances to form a more complex substance element +element = compound ex: Accordingly, the reactions are classified into different types: Reaction in which the more reactive element displaces the less reactive element.here li replace oh from water. Types of reactions include single displacement, double displacement, synthesis, decomposition and combustion. It is a redox reaction in. Published by rosaline floyd modified over 5 years ago. A chemical reaction is a process in which one or more substances it is a special type of double displacement reaction (a and b switch places) and these chemical. What is single displacement reaction?

Double replacement i say type of chemical reaction synthesis, decomposition, double or single k+b2o3=k2o+b.

The double displacement reaction is a pretty obvious extension of single displacement.

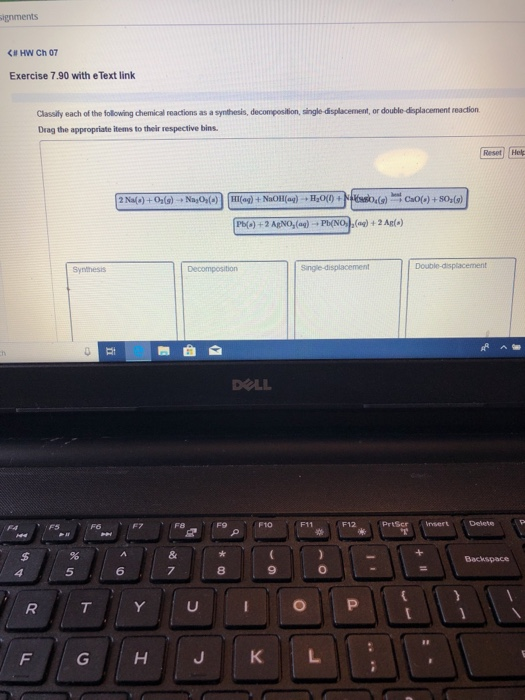
Classify the reactions as synthesis, decomposition, single replacement or double replacement, and write balanced formula equations.
This is most easily demonstrated with fluorine, chlorine, bromine, and iodine.
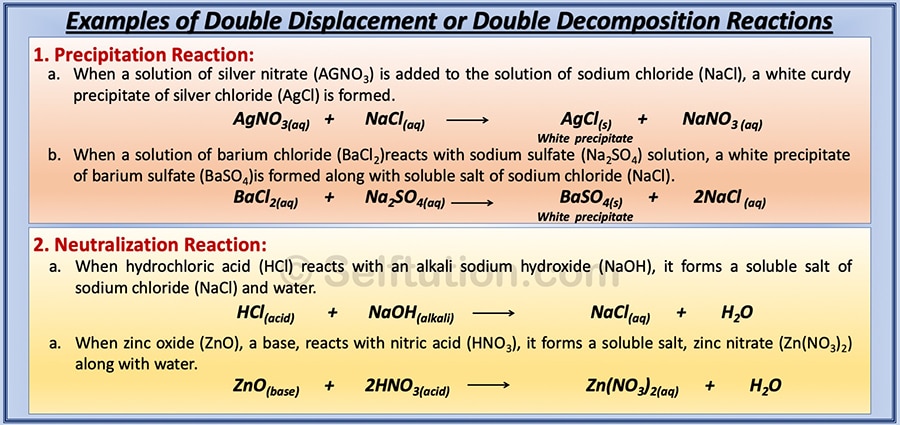
Synthesis, decomposition, synthesis, single replacement (also called single displacement) and double replacement (also called double displacement).
These are really just decomposition reactions;

Define all five reaction types.

Use the activity series to correctly predict many chemical reactions can be classified as one of five basic types.

These are really just decomposition reactions;
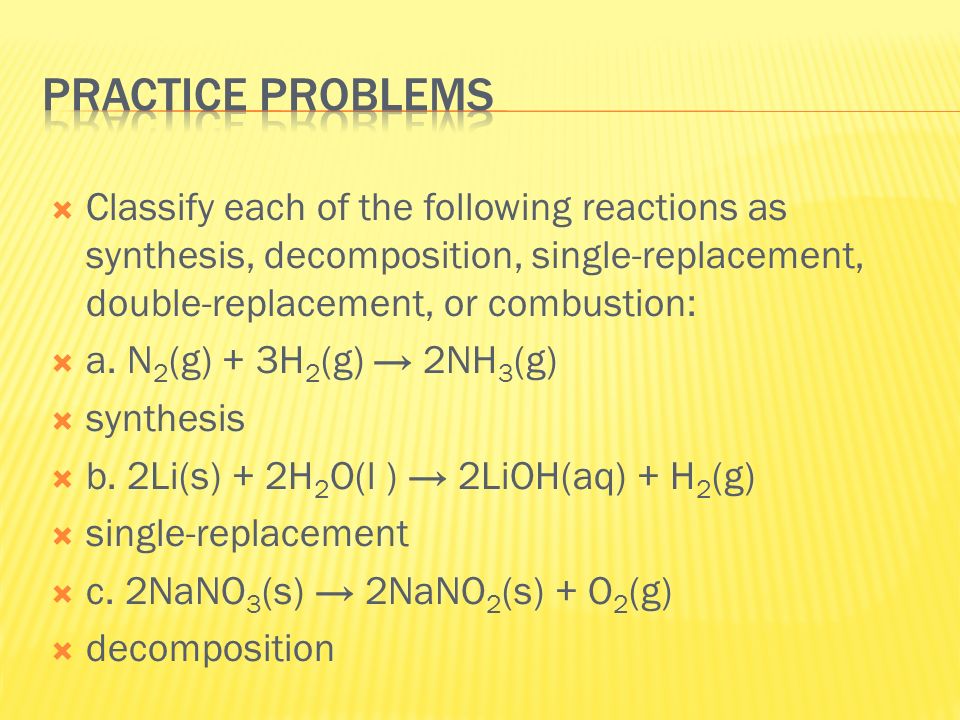
Types of reactions synthesis decomposition combustion single displacement double displacement.

Classify the reactions as synthesis, decomposition, single replacement or double replacement, and write balanced formula equations.
Chemists classify chemical reactions into different categories, and four of them include:

What type of reaction is this?
Reaction in which a single reactant decompose to give two or more products.
We'll learn about the five major types of chemical reactions:
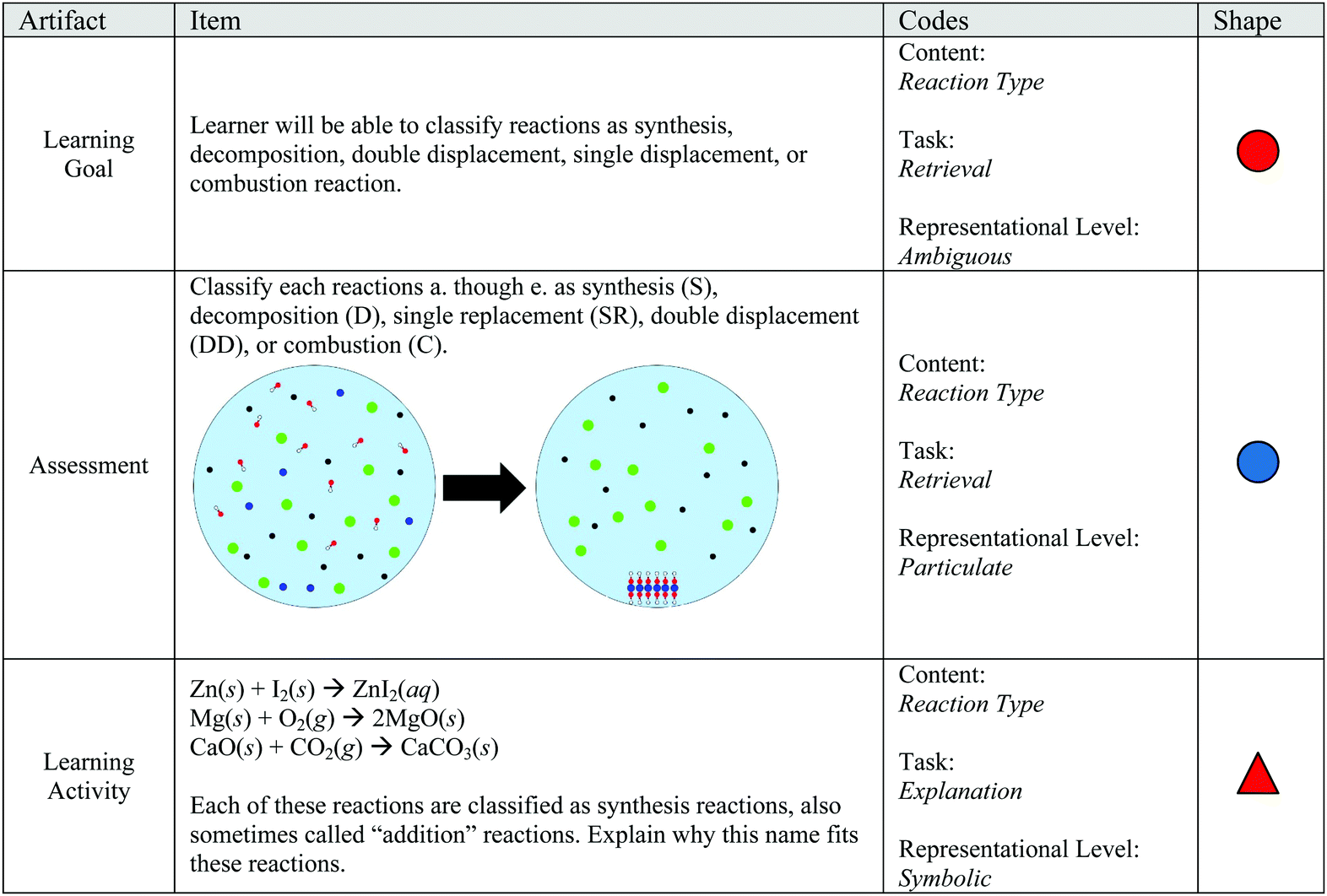
Published by rosaline floyd modified over 5 years ago.

Define all five reaction types.

Definition of single replacement (or single displacement) reactions.

A simple way of classifying chemical reactions is to group them in one of four basic types:

These are really just decomposition reactions;

For each of the following stations, you will complete the data table columns titled reactants, observations before reaction and observations.

H2o + h2 + o2 a.

Classify chemical reactions as one of these three types given appropriate descriptions or chemical equations.
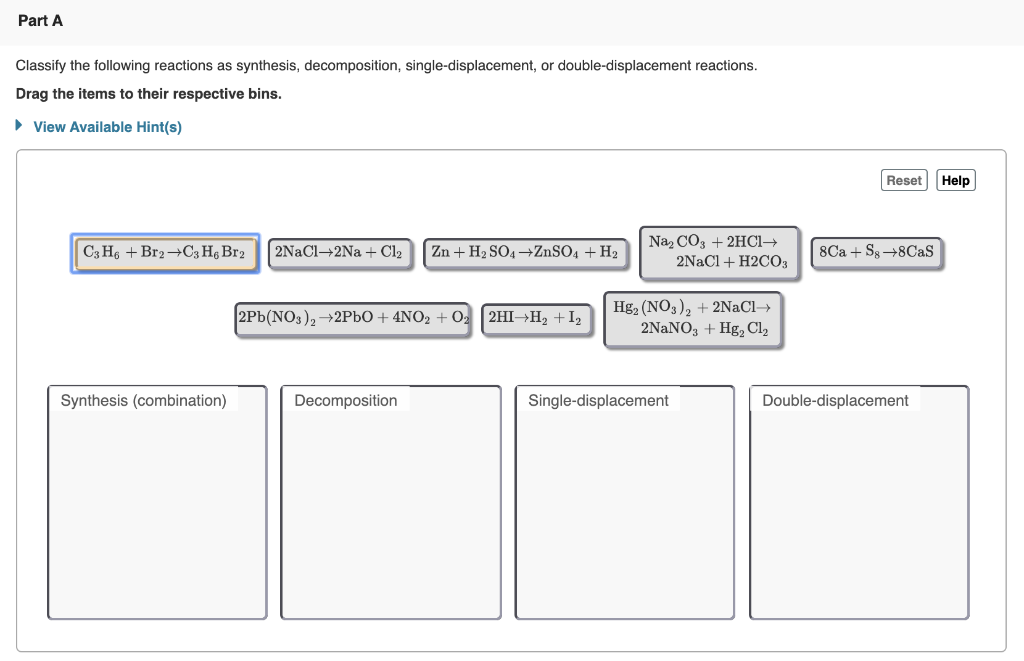
H2o + h2 + o2 a.

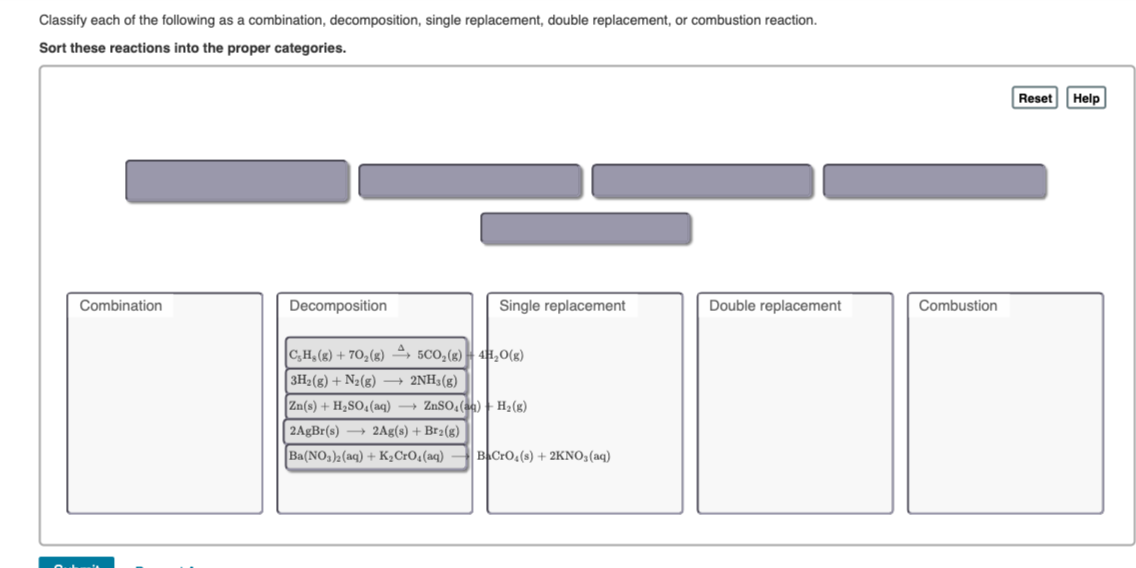
Types of reactions synthesis decomposition combustion single displacement double displacement.

Define all five reaction types.

Learn about displacement reactions topic of chemistry in detail explained by subject experts on vedantu.com.

Predicting and determining the products using the reactivity series.
/types-of-chemical-reactions-604038_FINAL-728e463b035e4cca84544ed459853d5c.png)
This is most easily demonstrated with fluorine, chlorine, bromine, and iodine.

Six types of decomposition reactions.

Learn about displacement reactions topic of chemistry in detail explained by subject experts on vedantu.com.

Synthesis, decomposition, single displacement, double displacement.
Predicting and determining the products using the reactivity series.
For example, the reaction below.
Use the activity series to correctly predict many chemical reactions can be classified as one of five basic types.
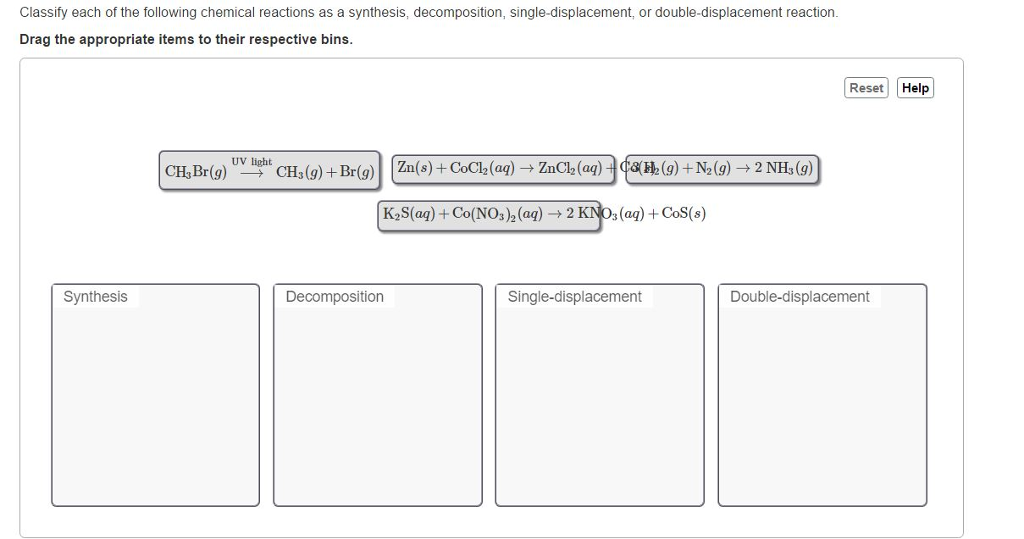
Synthesis, decomposition, single displacement, double displacement.

These are really just decomposition reactions;

1 814 328 просмотров 1,8 млн просмотров.

Posting Komentar untuk "Types Of Chemical Reactions Classify Each Of These Reactions As Synthesis, Decomposition, Single Displacement, Or Double Displacement. - Ch150 Chapter 5 Chemical Reactions Chemistry"